Key Insights:
- Syrup leads the crypto bubble with an impressive 305% performance.
- The protocol’s $2.5B TVL shows its increasing growth and stability.
- Syrup’s rise highlights the shift towards yield-generating DeFi protocols.
Major DeFi protocols, such as Syrup, have managed to experience a fantastic growth, leaving a signature of its own in the crypto bubble. As its price and total value locked (TVL) boomed, Syrup has become an evident powerhouse of the decentralized finance sphere.
These great statistics of the protocol show that it is well-performing and has a chance to become a leader on the market as we progress to the altcoin season.
A Surge in the Crypto Bubble
The price of Syrup reached all-time high (ATH) and it is a considerable addition to the current crypto bubble. The feat is supported by significant milestones, such as a second $100 million deposit and a TVL of $2.5 billion.
Syrup is also expected to record the highest monthly volume of revenue ever in its history thus consolidating its position as one of the major actors within the DeFi market.
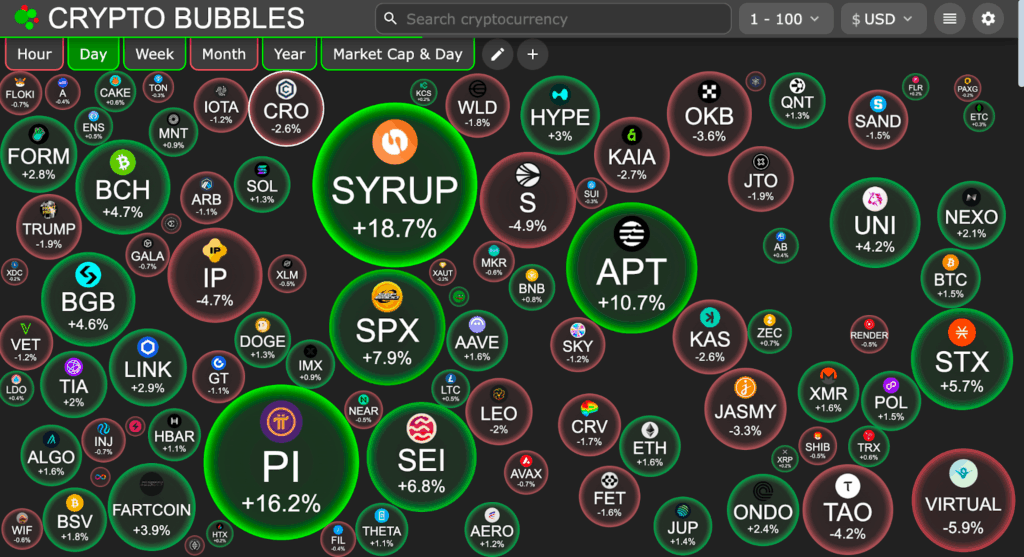
Syrup is among the most prominent performers in the context of the crypto bubble, with a daily rise of 18.7%. SPX (+7.9%) and PI (+16.2%) are also among the other assets with impressive increase, yet, Syrup is growing particularly impressive considering that it has a solid base and fast-growing audience.
This trend of the crypto bubble indicates a market that is experiencing an increase in assets and Syrup has been leading such an upsurge.
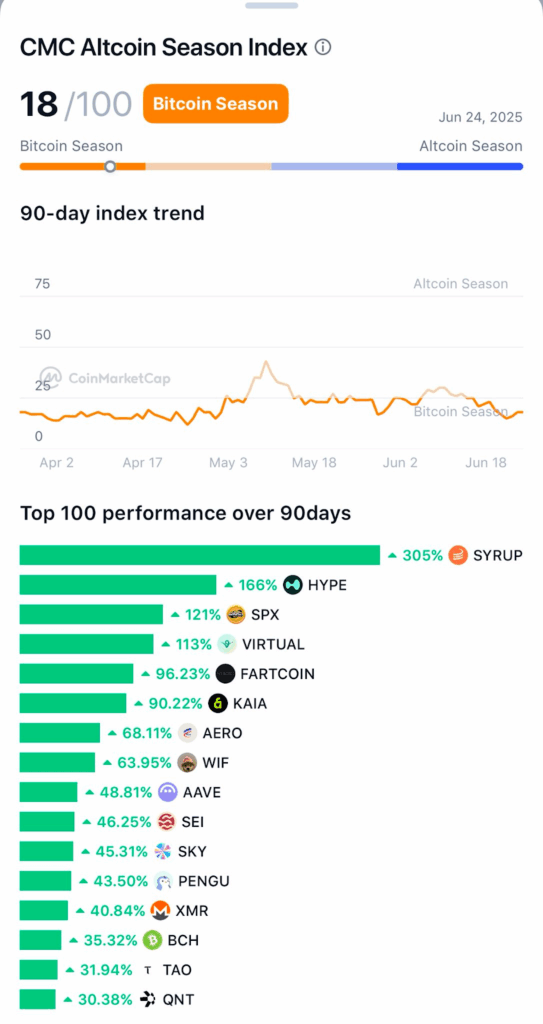
This remarkable performance of Syrup is worthy of mention but what is more important is that the inflated crypto bubble is still broadly growing, as altcoins are becoming more popular. The CMC Altcoin Season Index provides a scale of 0-100 with 0 indicating that we are still early in terms of the Bitcoin season as we only have 18/100.
Nonetheless, Syrup has already demonstrated itself as an outstanding asset with a stellar 305% showing in the last 90 days. With this performance it makes Syrup coin to be on top of the best 100 coin in the market and the altcoin season has not yet started.
The 90-day chart tracking the performance definitely shows that Syrup was dominating the crypto bubble. It is vastly beating its rivals, such as the altcoins like HYPE (+166%), and SPX (+121%), proving that the development of Syrup is not a temporary phenomenon, but a tendency.
This is reflective of Syrup as the currency also keeps rising indicating that it has even more to offer when the altcoin season really kicks off.
Real Yield and Sustainable Growth
The rise of Syrup is not only to take advantage of the speculative nature of the crypto bubble but it is underpinned by actual yield, good infrastructure and definite growth opportunities.
With additional capital entering Syrup, we can see that its TVL is also increasing, proving that users and investors are getting more optimistic in the capacities of the protocol to make profits.
Such tendency to change towards yield-generating assets is typical of the new environment in the crypto bubble where sustainability of growth becomes one of the priorities of investors.
When it comes to the monthly protocol income, Syrup may be on the verge of having the largest income to date. The second deposit of 100 million dollars is another indication of how Syrup is scaling up and becoming increasingly liquid.
Syrup is also gaining popularity and funds by the day, which makes its leadership in the crypto bubble impossible to deny.
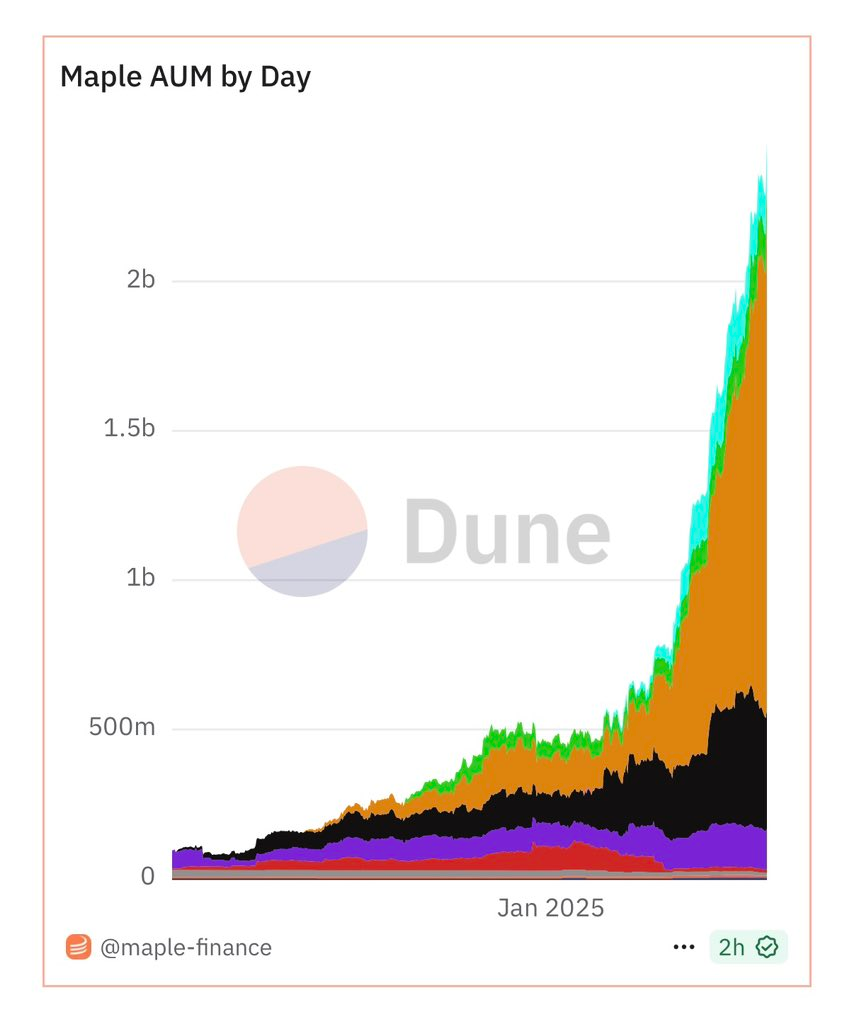
According to a TVL growth chart of Syrup, one can only observe that the protocol is growing fast, with revenues jumping through the roof in recent months.
This chart, combined with the crypto bubble indicators, points to the movement of Syrup that reflects what happens in the DeFi market in general, where protocols with real yields and novel infrastructures are experiencing a rapid TVL expansion.
The fact that syrup has dominated the crypto bubble is a clear testimony that the protocol is very strong and has more potential to grow. Having an ATH price, and TVL of $2.5 billion with an on-target revenue, Syrup can dominate the DeFi ecosystem as the alternative coin season unfolds.
With the general crypto bubble constantly expanding, Syrup remains among the most thrilling assets to observe within the following months.
